A new direct approach to pancreatic cancer therapy and other solid tumours.
The most common form of radiotherapy, Stereotactic Body Radiotherapy (‘SBRT’) involves focussing external radiation into tumour(s) in the body. However, collateral damage is imparted to healthy tissue in the path of the beams and organ movement significantly limits the administered dose. More advanced Proton Beam Radiotherapy (‘PBRT’) greatly reduces collateral damage by employing proton beams of fixed penetration depth but still face the challenge of organ movement, however, its widespread adoption is heavily impaired by the cost of building treatment suites and low patient throughput. As such, these approaches are seldom used to treat abdominal organs such as the liver and pancreas.
Introducing YntraDose designed as a safe, easy-to-use, targeted and personalised radiotherapeutic treatment option for a wide range of solid tumour cancer anatomies and patient groups that overcomes the limitations of SBRT and PBRT whilst maximising the administered radiotherapeutic index for the patient in a single day case treatment session.
YntraDose consists of radioactive microspheres (that contain beta-radiating yttrium-90 (90Y)) and a biocompatible bioglue agent that, following injection, rapidly sets to form a polymerized matrix that retains the microspheres specifically at the site of injection. Depending on the clinical setting, YntraDose can either be percutaneously delivered directly to the tumour under radiological guidance or directly into a tumour bed following resection. Percutaneous administration is performed under local anaesthesia and conscious sedation by an interventional radiologist.
The impact of the clinical benefits for patients thanks to this new personalised radiotherapeutic treatment option are potentially broad.
Thanks to the targeted and localized direct intratumoural application of YntraDose, not only is the product relatively simple to use, but more importantly, there is the opportunity to reach lesions in a wide range of anatomies and therefore open the door to benefit many solid tumour cancer patient groups worldwide.
 Prof. Jafar Golzarian
Prof. Jafar Golzarian At the very beginning of the history of Interventional Oncology, ethanol injection, the only modality available at that time, was rather impressive, given that with simple percutaneous injections it allowed to achieve outcomes comparable to those of complex surgical procedures, albeit in selected cases. The new technology of YntraDose, using Y-90, seems to be the natural evolution of the old ethanol injection, but applicable in a wide range of neoplastic diseases of different organs, with single applications and greater precision of guidance, given the great progress of imaging modalities. In summary, an actual ‘targeted therapy’ extensively feasible.
 Prof. Luigi Solbiati
Prof. Luigi Solbiati The YntraDose Solution comprises three key product components:
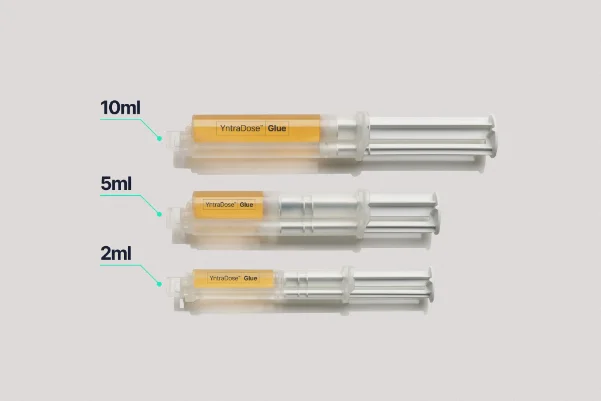
YntraDose®Glue
YntraDose-Glue – A sterile biocompatible bioglue provided as either a 2ml, 5ml or 10ml pre-filled double barrel syringe.
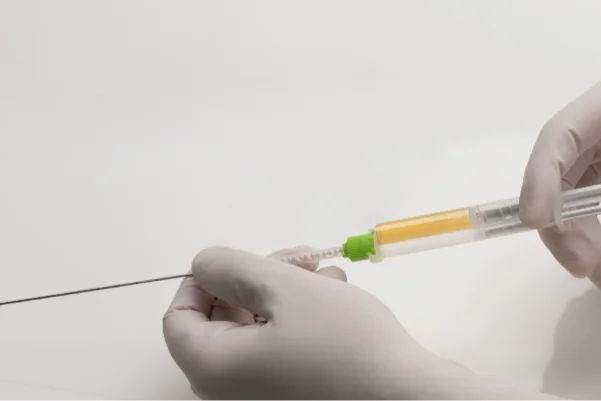
YntraDose®Deliver
YntraDose-Deliver – A sterile ‘mixing tip’ and introducer/needle.
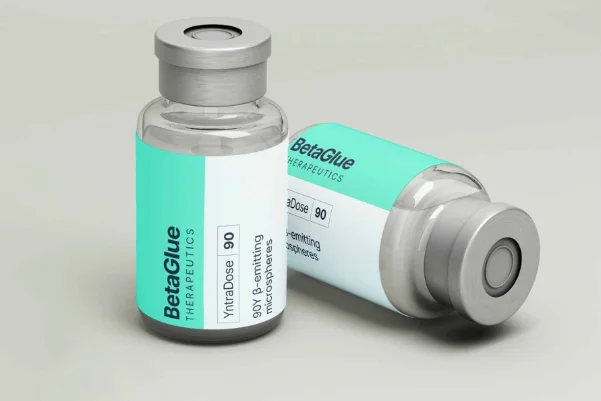
YntraDose®90
YntraDose-90 – A sterile vial containing a fixed dose and volume of Yttrium-90 microspheres.
How YntraDose works
By working closely with our clinical advisors, the YntraDose Solution has been carefully designed to provide a convenient easy-to-use and localized radiotherapy treatment for solid tumours that can be used as add-on to Standard of Care therapies.
Tailored for use by Nuclear Physicians and Interventional Radiologists, YntraDose allow to provide patients with a personalised, targeted and precise radiotherapy treatment that can be achieved on a tumour-by-tumour and patient-by-patient basis.
01Plan
Red circle showing pancreatic porcine splenic lobe.
02Prepare
YntraDose-90 added to the YntraDose-Glue syringe.
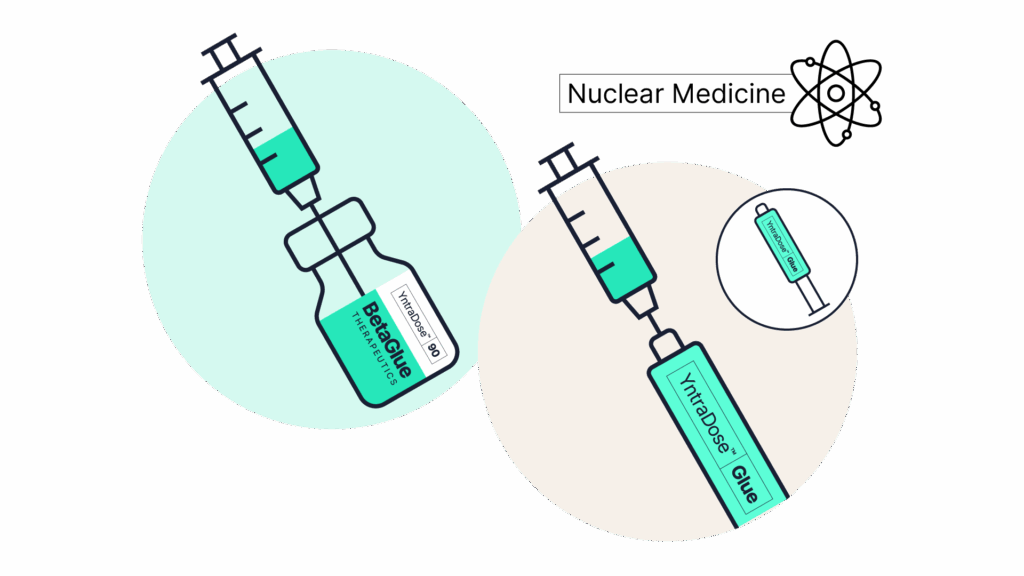
03Treat
Attached YntraDose-Deliver injection system to YntraDose Syringe.
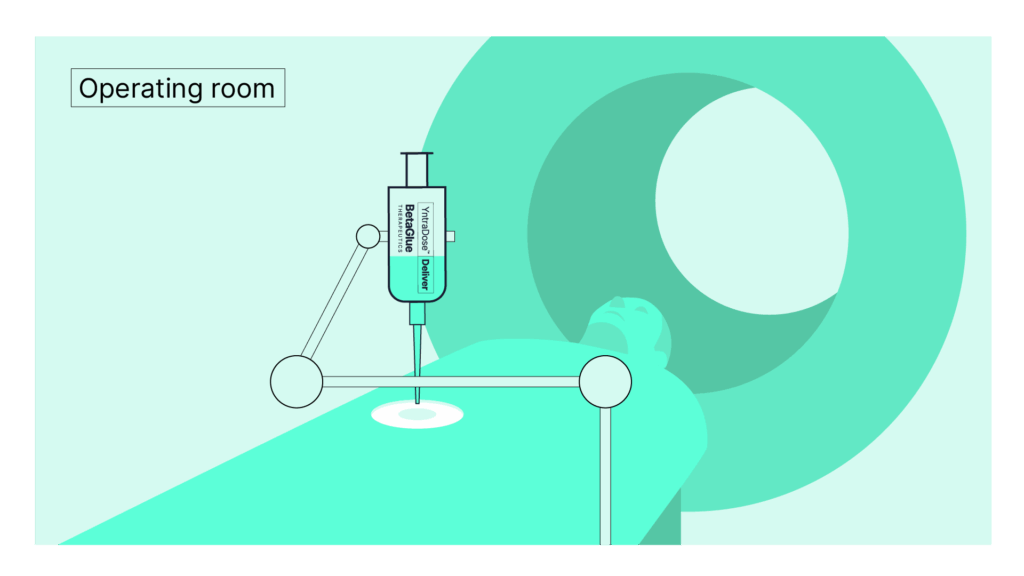
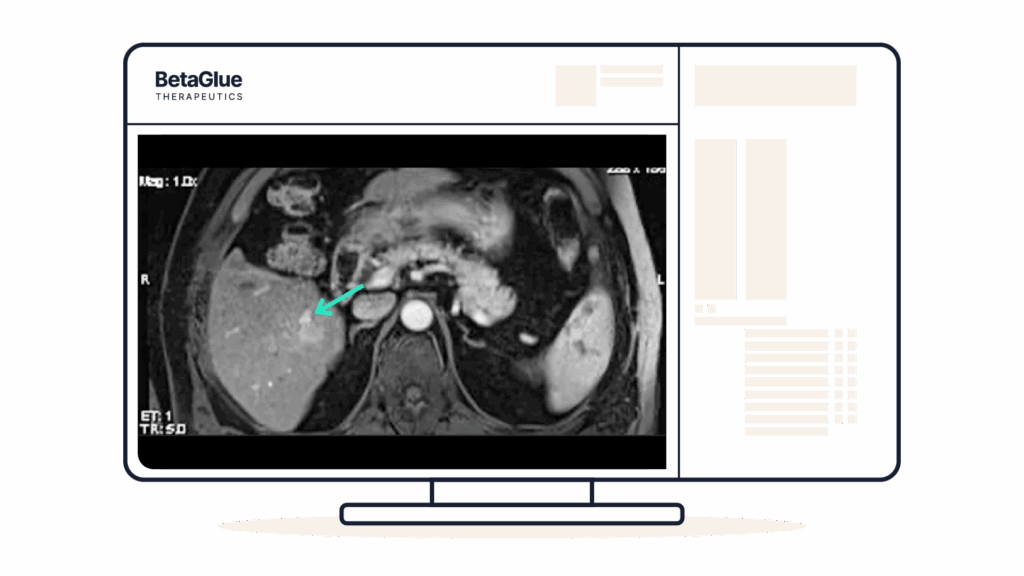
04Assess
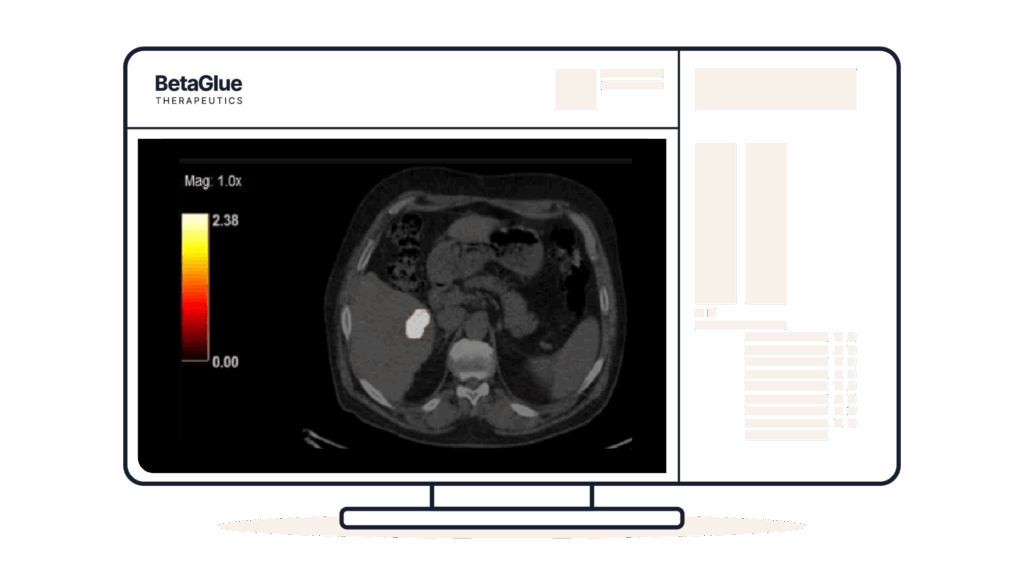
Y90-PET CT showing focalized signal of the implantation of YntraDose.
Publications Pre-Clinical and Clinical
Pre-Clinical
- Modelling a new approach for radio‑ablation after resection of breast ductal carcinoma in‑situ based on the BAT‑90 medical device.Scientific Reports | (2022) 12:14
- A Novel Injectable Hydrogel Matrix Loaded with 90Y Microspheres for the treatment of Solid Tumours. Amato et. al. Anticancer Research42:827-836 (2022).
- In-vivo models for the performance and safety of BAT-90, a novel 90-Yttrium-based internal radiotherapy platform. Amato et. al. In Vivo36:2052-20 (2022).
- Preclinical study of safety and efficacy in vivo of a yttrium-90 resin microspheres glue formulation in a large animal model of pancreatic cancer. Journal of Clinical Oncology Volume 43, Number 16_suppl 2025
- Clinical Percutaneous image-guided radio-ablation using BAT-90 – initial results of ongoing first-in-man clinical trial.Mizandari et. al. JVASC Interv Radiol 43:e29-e70 (2023).
Advantages of YntraDose®
For users (nuclear physicians & interventional radiologists)
Easy to Use: YntraDose consists of a single percutaneous injection administered under CT guidance, delivering a fixed volume and dose to pancreatic lesions classified as unresectable locally advanced pancreatic ductal adenocarcinoma (uLA-PDAC). YntraDose is used as an adjunctive therapy in combination with standard of care (SOC) treatments such as FOLFIRINOX or gemcitabine/nab-paclitaxel
For patients
Safe: YntraDose is designed to avoid the need for general anaesthesia, to eliminate off-target radiation and with the ability to treat tumours near delicate organ structures
Convenient: Radiotherapy provided as a single day-case procedure as an outpatient
Minimally Invasive Procedure: Simple administration using an introducer/needle
For healthcare providers
Accessible: Provides a radiotherapeutic treatment option in unresectableLocally Advanced PDAC where there is a huge unmet medical need
Cost Effective: No need for capital equipment and procedures are ‘non-DRG’ day-case

Intellectual property
BetaGlue Medical possesses a wholly owned intellectual property estate across multiple jurisdictions. In addition to both granted and pending composition of matter, product by process and medical use patent claims, the company also owns a significant body of know-how. BetaGlue Medical, and YntraDose are registered trademarks of BetaGlue Technologies SpA.

