PDAC
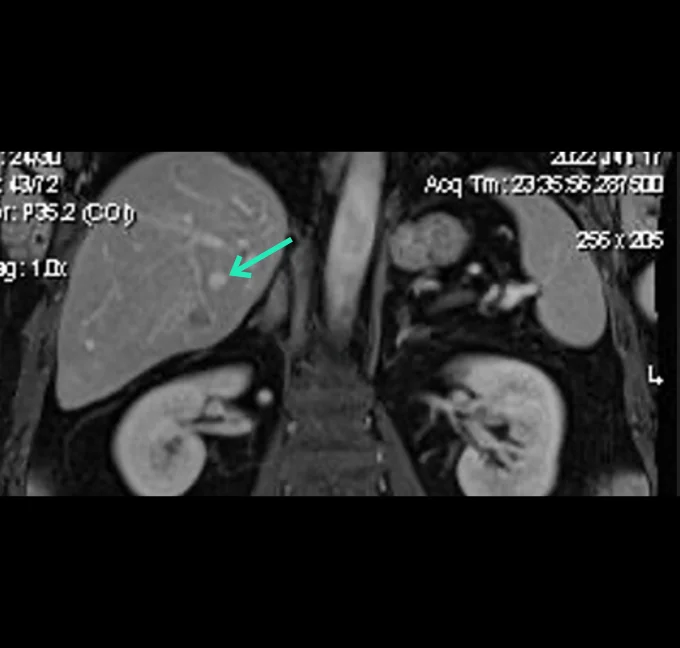
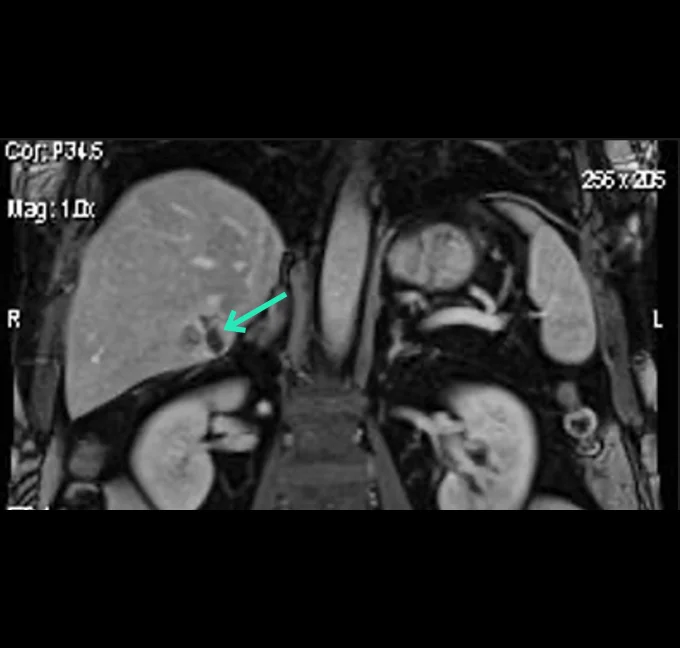
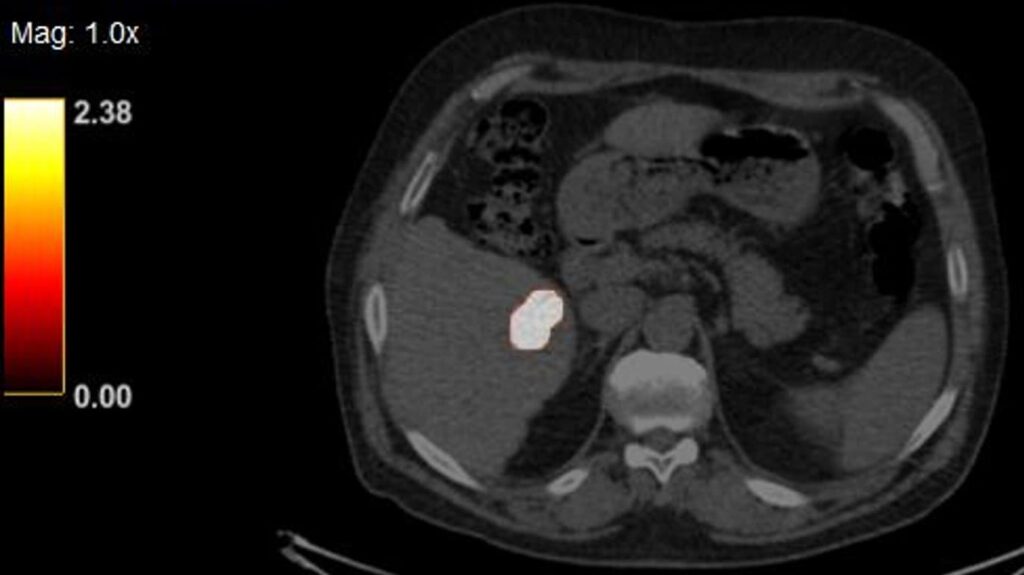
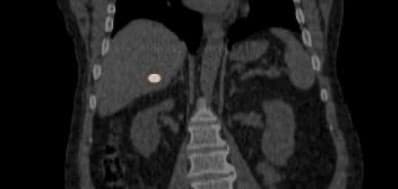
Betaglue Therapeutics is committed to pursue as lead indication Pancreatic Adenocarcinoma cancer, considering PDAC has one of the worst forms of cancer in terms of progression-free and overall survival and is the seventh most common cause of cancer related death worldwide. Around thirty percent of PDAC patients are considered as locally advanced cancer patients.
BetaGlue Therapeutics successfully completed all preclinical studies for this indication and is ready to start an Early Feasibility Study (EFS) in pancreatic cancer patients in Q1 2026.
Clinical Data HCC (Hepatocellular Carcinoma)
YntraDose (previously called BAT-90) has been studied in a FIH Trial to assess the performance of BAT-90 during the percutaneous ablative procedure of resectable and unresectable primary liver lesions (HCC). The study indicated that No treatment related AEs and ADEs limiting the ability to deploy YntraDose occurred and 100% of the lesions treated showed to have been reached by the product.
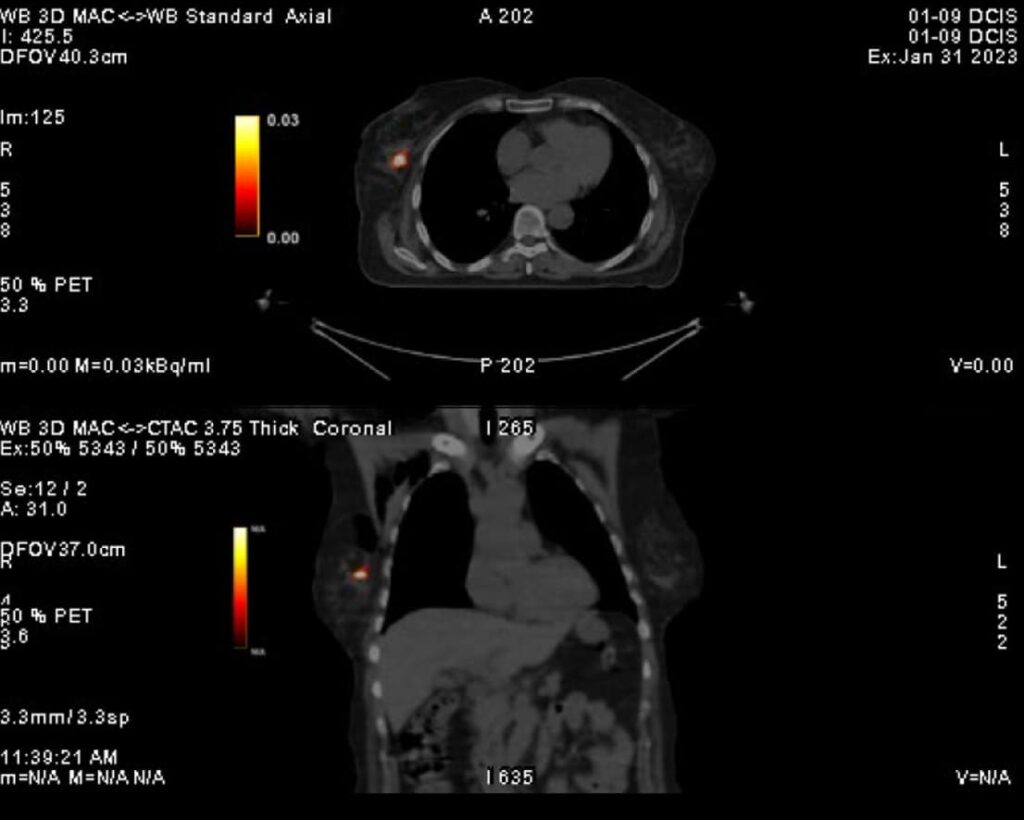
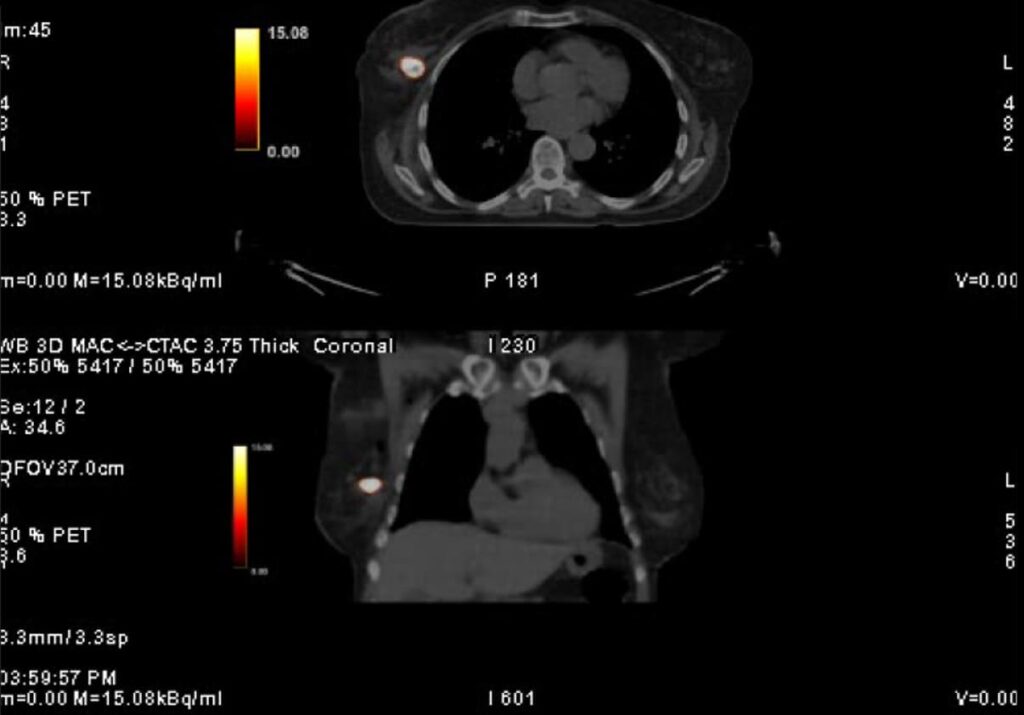
Clinical Data DCIS (Ductal Carcinoma In Situ)
YntraDose (previously called BAT-90) has been studied also as treatment for radio-ablation of surgical margins following resection of breast tumoral lesions Stage 0/1/2A of DCIS (ductal carcinoma in situ) in a First in Human clinical investigation. The formal Data monitoring committee (DMC) held confirmed the safety profile of the treatment indicating that the device demonstrated an acceptable acute safety profile with no serious adverse events/serious adverse device effects during the follow-up period.
The impact of the clinical benefits for patients thanks to this new personalised radiotherapeutic treatment option are potentially broad.
Thanks to the targeted and localized direct intratumoural application of YntraDose, not only is the product relatively simple to use, but more importantly, there is the opportunity to reach lesions in a wide range of anatomies and therefore open the door to benefit many solid tumour cancer patient groups worldwide.
 Prof. Jafar Golzarian
Prof. Jafar Golzarian At the very beginning of the history of Interventional Oncology, ethanol injection, the only modality available at that time, was rather impressive, given that with simple percutaneous injections it allowed to achieve outcomes comparable to those of complex surgical procedures, albeit in selected cases. The new technology of YntraDose, using Y-90, seems to be the natural evolution of the old ethanol injection, but applicable in a wide range of neoplastic diseases of different organs, with single applications and greater precision of guidance, given the great progress of imaging modalities. In summary, an actual ‘targeted therapy’ extensively feasible.
 Prof. Luigi Solbiati
Prof. Luigi Solbiati Publications
- Modelling a new approach for radio‑ablation after resection of breast ductal carcinoma in‑situ based on the BAT‑90 medical device.Scientific Reports | (2022) 12:14
- A Novel Injectable Hydrogel Matrix Loaded with 90Y Microspheres for the treatment of Solid Tumours. Amato et. al. Anticancer Research42:827-836 (2022).
- In-vivo models for the performance and safety of BAT-90, a novel 90-Yttrium-based internal radiotherapy platform. Amato et. al. In Vivo36:2052-20 (2022).
- Preclinical study of safety and efficacy in vivo of a yttrium-90 resin microspheres glue formulation in a large animal model of pancreatic cancer. Journal of Clinical Oncology Volume 43, Number 16_suppl 2025
- Clinical Percutaneous image-guided radio-ablation using BAT-90 – initial results of ongoing first-in-man clinical trial.Mizandari et. al. JVASC Interv Radiol 43:e29-e70 (2023).